Research
Overview
Metastases are the main cause of human cancer deaths. As cancer progresses, cancer cells migrate from the initial tumor mass and spread to vital organs. The microenvironment surrounding the tumor is complex, and we are becoming increasingly aware that the microenvironment contains signals that regulate cancer cell behavior. We seek to understand what these signals are, which cells send them, and how this communication regulates cancer decision-making in complex environments. While these questions are deeply rooted within a cancer context, our projects also address fundamental aspects of cellular behavior.
Projects in the Lab
-

Lateral transfer of macrophage mitochondria to cancer
-

Macrophage differentiation and cancer proliferation
-

Regulation of cell-matrix interactions in vivo
-

Evolutionary origin, composition and function of cell-substrate adhesions
-

Development of chimeric antigen receptor macrophages (CAR-Ms) to target melanoma in vivo
Lateral transfer of macrophage mitochondria in cancer
(Taylor Stevens and Noah Bressler)
Alumni: Joey Casalini & Chelsea Kidwell
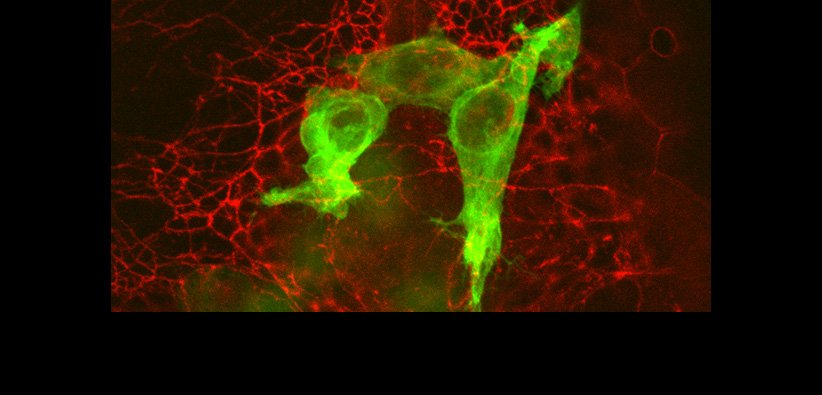
Macrophages are a component of our immune system, but have a paradoxical role in cancer – Macrophages can promote metastasis. We previously discovered that macrophages spill their internal contents into cancer cells during metastasis, but what is the functionally important information in the contents? We recently discovered that macrophages transfer an organelle called mitochondria directly to cancer cells, and that receipt of macrophage mitochondria allow cancer cells to grow more rapidly. Thus, we seek to answer how this mitochondrial transfer process occurs and what happens when the mitochondria are received by cancer cells in an effort to develop strategies to prevent this process. Surprisingly, we find that after mitochondrial transfer occurs, the transferred mitochondria remain as a spatially distinct population from the host mitochondrial network. Furthermore, we found that high levels of local reactive oxygen species accumulate at transferred mitochondria, suggesting an intriguing hypothesis that transferred mitochondria may provide a signal to tumor cells, rather than providing excess mitochondrial function as has been previously described. Check out the paper for more info:
https://pubmed.ncbi.nlm.nih.gov/36876914/
We are now extending these studies to ask how cells identify and manage distinct mitochondrial pools as well developing a new system to detect cells that have received mitochondria with high sensitivity.
Legend: Left: Graph of cell confluence over time on the Incucyte. Right: Quantification of cell confluence at 72 hours.
Macrophage differentiation and cancer proliferation
(Sophia Varady and Daniel Greiner)
Macrophages exhibit enormous plasticity and can differentiate into a variety of subtypes, with each subtype performing a specific function. The canonical understanding for macrophage function is that macrophages differentiated into a “pro-tumor state” promote cancer cell proliferation, whereas macrophage differentiated into an “anti-tumor state” inhibit cancer cell proliferation. We have discovered that contrary to this paradigm, macrophages differentiated into either “pro-tumor” or “anti-tumor” both inhibit cancer cell proliferation compared to cancer cells alone. You can find more information about this work in the following preprint:
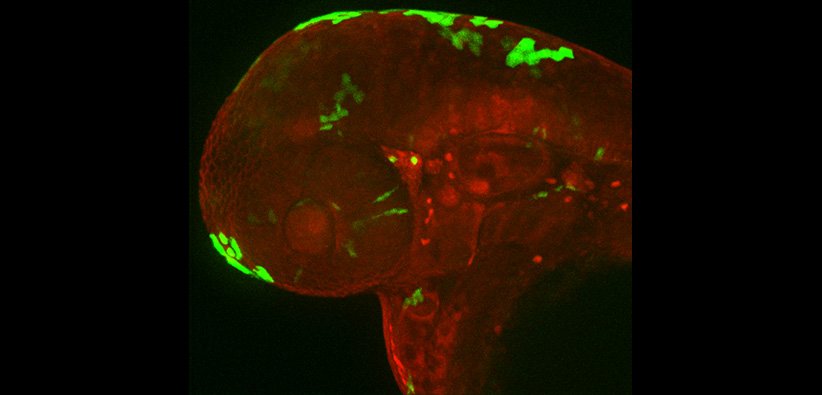
Legend: Image of zebrafish melanoma cell migrating in a zebrafish larvae. Focal adhesions are Paxillin-positive (green) and colocalize with actin (magenta).
Regulation of cell-matrix interactions in vivo
(Mackenzie Roman and Kara Jones)
Alumnus: Qian Xue
It is unclear how a melanocyte transitions from a premalignant nevus to an invasive melanoma cell; even less studied is the direct examination of this transition to motility in vivo. On 2-dimensional substrates, cancer cells migrate through a net forward movement of cell protrusion at the front and cell retraction at the back. Central to this form of cancer cell migration is cell attachment to the underlying substrate through focal adhesion complexes. Despite years of research on focal adhesion formation, the function and organization of these structures in their native environment are still unclear. What is the composition and dynamic regulation of focal adhesion structures in vivo? How do surrounding cells and extracellular matrix composition affect focal adhesion assembly and function in cancer cells? Inhibitors targeting focal adhesion components are currently being explored in the clinic, thus it is critical to understand how these complexes function in cancer cell migration in animals. We have developed an innovative system in zebrafish larvae in which we can directly visualize the formation of focal adhesion structures in highly migratory melanoma cells on a relatively planar surface of the larval zebrafish skin. We have used this system to dissect the composition and dynamics of focal adhesion structures in vivo and have identified unique properties of focal adhesion formation and regulation in cancer cells in their native environments. For more information on how focal adhesions are different in vivo versus in cell culture, check out our paper:
https://pubmed.ncbi.nlm.nih.gov/36723624/
We are now testing the function and dynamics other focal adhesion proteins and using a combination of animal models and proteomics to further dissect post-translational modifications.
Evolutionary origin, composition, and function of cell-substrate adhesions
(Julio Fierro)
Focal adhesion machinery was initially thought to be animal (metazoan)-specific, however comparative genomics of organisms evolutionary distant from Metazoa suggest full-length homologues of core components are found in organisms as distant as Amoebozoa. Interestingly, many organisms evolutionarily distant from Metazoans form cell-substrate adhesions for migration despite lacking the complete focal adhesion machinery found in Metazoans. We hypothesize species evolutionarily distant from Metazoans form cell-substrate adhesions using focal adhesion molecule orthologues that maintain conserved adhesive functions and interactions. We use evolutionary analyses, biochemistry, and cell biology to elucidate the composition and function of evolutionary distant cell-substrate adhesions and characterize adhesion orthologues in the Amoebozoan, Dictyostelium discoideum. Check out our paper for more information on the conserved functions of PaxillinB, a homologue of the vertebrate Paxillin.
Legend: CSPG4-expressing CAR-macrophages (green) attached onto a melanoma spheroid (magenta) in 3D.
Development of chimeric antigen receptor macrophages (CAR-Ms) to target cancer in vivo
(Daniel Greiner and Elena Kurudza)
Alumnus: Trinity Waddell
The growing list of tumor-promoting macrophage functions includes increasing proliferation, increasing angiogenesis, facilitating immune escape, and strengthening metastatic potential. The recent development of chimeric antigen receptor macrophages, CAR-Ms (modeled off of the successful CAR-T cell therapy), to kill cancer cells through phagocytosis and reprogramming of the tumor immune environment has opened doors for new therapeutic cancer interventions. We have generated CAR-Ms targeting CSPG4/NG2, an antigen expressed in several cancers including melanoma and glioblastoma. CSPG4/NG2-targeting CAR-Ms phagocytose cancer cells in vitro and inhibit cancer spheroid growth in 3D. CSPG4/NG2-targeting CAR-Ms also inhibit melanoma growth in vivo. For more information, check out the following preprint:
https://www.biorxiv.org/content/10.1101/2024.06.04.597413v1
We are currently building second generation CAR-Ms, as well as moving this system into melanoma brain metastases and animal models of glioblastoma.